Posted by: Nerys Setija
Burden of disease
Schistosomiasis is a major health problem. The parasite exists in tropical and subtropical areas, mostly poor communities with inadequate sanitation. 779 million people are estimated to be at the risk of infection, of which 85% are in Africa. More than 200 million people in 74 different countries are infected with schistosomiasis, and 120 million of these people have developed the disease. (1) This leads to the loss of an astonishing 1.53 million DALY’s. Mortality estimated to vary between 25 000 and 200 000 per year. (2) These numbers show the high impact of schistosomiasis on both health and socioeconomics of these areas.
Besides this, the infection rate is highest in children between 5 and 17 years of age. They possess a higher risk than adults because of poorer hygiene, more frequent contact with water and lack of knowledge about the disease. For example bathing and swimming in dams and rivers, crossing rivers on the way to school barefooted, unhygienic use of toilets and unprotected water sources. (1) The fact that it’s mostly children that get infected contributes to the relevance of a vaccine.
Microorganism and its Lifecycle
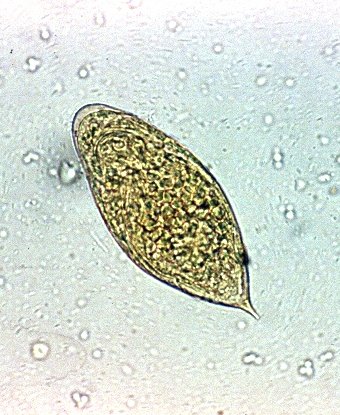
Schistosomiasis is a disease caused by helminths of the genus Schistosoma. There are multiple species, differently distributed around the world. The main disease causing species are S mansoni, S haematobium and S japonicum. The eggs of the S mansoni species are recognized during microscopy by their lateral spine and the S haematobium eggs are recognized by their terminal spine. S japonicum have more of a round form with a small lateral spine. (3)
Source: Gryseels et al. (2006)
Intermediate hosts are freshwater snails. Each different schistosoma has their own specific freshwater snail as their host. S mansoni is transmitted by Biomphalaria snails (middle), S haematobium is transmitted by Bulinus snails (left) and S japonicum is transmitted by the Oncomelania snail (right). (3)
Bulinus truncatus, Biomphalaria glabrata and Oncomelania hupensis in a lab. Source: Lewis, Liang, Raghavan, Knight (2008)
For the majority of their lifecycle the parasites are asexual. Sexual reproduction only occurs in the stage where fullgrown helminth have developed in the human body. To reproduce the male’s body forms a channel in which it holds the thinner female. (3) This means that both sexes are needed in order for the female to produce eggs. Vaccines could focus on limiting the worm's ability to mate which would lead to a decrease in egg production.
Schistosomiasis embraced male and female worms. Image credit: Trustees of the Natural HIstory Museum, London. https://www.eliminateschisto.org/working-together/schistosomiasis
In order to explain the reproduction of Schistosoma we will explain the lifecycle of the microorganism in different steps. (3)
The schistosomes (matured male and female worms) live as embraced couples within the human body. The female produces eggs that are excreted in the urine or feces.
The eggs can survive for up to 7 days. On contact with water the eggs can release the miracidium. The miracidia must invade the snails within 24 hours before they die, but they are guided by sunlight. The eggs will therefore only release the miracadia during the day.
When the miracadia have penetrated the snails they start to develop into sporocysts. Later the sporocysts will develop into swimming cercariae (larvae) with a tail.
4-6 weeks after infection of the snail the cercariae are released into the water and have up to 72 hours to find a host.
They penetrate the skin of a human, during which they lose their tail, and migrate in the blood via the lungs to the liver. In the human body they become schistosomula.
They mature to male and female schistosomes in the portal vein for 4-6 weeks and migrate to the perivascular or mesenteric venous plexus where they feed on blood.
Schistosomes employ multiple tactics to evade the host’s immune system in order to develop further through its life cycle. Vaccines could be targeted against these immune evasions tactics like explained in this post.
| Schistosomiasis Life Cycle. CDC Centers for Disease Control and Prevention. https://www.cdc.gov/parasites/schistosomiasis/biology.html |
The pathology of schistosomiasis is mainly due to granuloma formation by the eggs. The eggs get stuck in tissues because of their spikes, this leads to granuloma formation. This can cause liver fibrosis and portal hypertension.
There is an acute and a chronic form of Schistosomiasis. Acute schistosomiasis mainly affects travelers with a primary infection, who then develop a feverish syndrome named Katayama fever. A systemic hypersensitivity reaction occurs because of the migrating schistosomula. The cercariae can provoke a urticarial rash or a swimmers’ itch.
Chronic schistosomiasis is mostly seen in individuals living in the poor endemic areas who then suffer from long-standing infections. Lesions are due to eggs trapped in tissues secreting proteolytic enzymes.
On top of the acute and chronic clinical pictures, different species cause different forms of schistosomiasis. S haematobium lives in the perivascular plexus of the bladder and causes urogenital schistosomiasis. S. Mansoni and Japoni live in the mesenteric plexus of mostly colon and rectum and cause intestinal schistosomiasis. (3) The symptoms are described in the figure below.
Diagnosis and Control
There are different diagnostic techniques to detect schistosomiasis infection. We will explain to you the use of microscopy, serology, PCR and urine strips.
Microscopic detection of parasite eggs in stool or urine is the gold standard for the diagnosis of schistosomiasis. In urogenital schistosomiasis this includes a filtration technique with nylon, paper or polycarbonate filters. In intestinal schistosomiasis a Kato-Katz technique (methylene blue-stained cellophane soaked in glycerin or glass slides) is used. A disadvantage of microscopy is that it’s only applicable 6-8 weeks after infection. Early infections with low egg counts will be missed.
Serology is based on the detection of antibodies in blood or urine samples. This technique can diagnose infection without the need of egg production by mature worms. It can be used in the early phase to show exposure to infection and it is primarily used in travelers. Serology is sensitive but difficult to apply in field conditions. Also it cannot distinguish between active infection or history of exposure. (3)
PCR is the most sensitive method to detect infection but it is only available in specialized and academic laboratoria. This is clearly not used in endemic areas, but only in Western countries for the detection of schistosoma in travelers. (4)
Urine reagent strip assays are based on the detection of antibodies in the urine. The test is rapid and requires no technical equipment or training. Therefore it is easy to apply in the field of endemic countries. The disadvantage of the technique is that it has a higher risk of false positives and negatives. (5)
Prevention and control of the disease is nowadays based on reducing disease with large scale population treatment with praziquantel. Other safety measures include access to safe potable water, adequate sanitation and snail control. To reduce the risk in children specifically, studies are performed to give hygiene education wit (1)
Praziquantel is a chemotherapy used as both treatment and prophylaxis. Groups at risk targeted for preventive treatment are preschool/school-aged children, adults in endemic areas, people with occupations such as fishermen, farmers, irrigation workers. The cure rate increases with the given dose. A meta-analysis (6) showed a protection rate of 52% at 40mg/kg up to a protection rate of 91% at 80mg/kg. Data of 2019 shows that 44.5% of people requiring treatment were reached for treatment globally. These results only drive home the point that a vaccine is absolutely necessary.
If you have any comments or remarks please leave them below! We are always eager to hear how we can improve our blog or learn about any new developments.
References
- Hajissa K, Muhajir A, Eshag et al. Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC Research Notes. 2018 Oct 31; 11: 779. doi: 10.1186/s13104-018-3871-y
- World Health Organization. Schistosomiasis. [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- Gryseels B, Polman K, Clerinx J et al. Human Schistosomiasis. The Lancet. 23–29 September 2006, Pages 1106-1118. doi: 10.1016/S0140-6736(06)69440-3
- RIVM. Schistosomiasis richtlijnen. [Internet]. Available from: https://lci.rivm.nl/richtlijnen/schistosomiasis
- van Dam G, Wichers J, Falcao Ferreira T et al. Diagnosis of Schistosomiasis by Reagent Strip Test for Detection of Circulating Cathodic Antigen. Journal of Clinical Microbiology. 2004 Dec; 42(12): 5458-5461. doi:10.1128/JCM.42.12.5458-5461.2004
- Liu R, Dong H, Guo Y et al. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis. 2011 Oct 17;4:201. DOI: 10.1186/1756-3305-4-201
